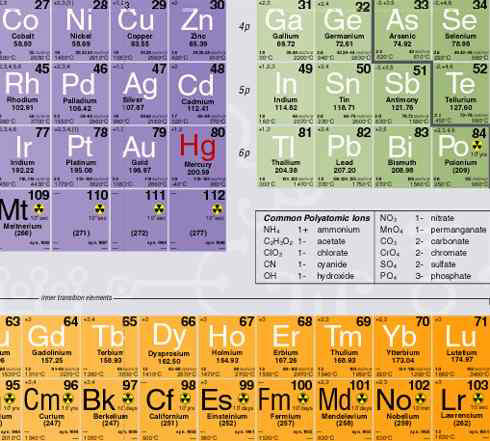
The information is present in a clear way, along with the color scheme which highlights group similarities and s-, p-, d- and f-blocks of elements. The color darkens as the atomic number goes up in a period to indicate an increasing electron count. The groups have identical colors to thereby show behavioral (and valence) similarities. Further, the table shows the suborbital level that is being filled (5p beside Indium) reinforcing students' understanding of electron configurations.